HydroFLOW Applications – Cooling Towers, Boilers and Other Equipment Protection
HydroFLOW is a unique water conditioning device that is capable of providing many benefits. The reduction and prevention of scale build-up in many process industries and HVAC systems are not the only areas HydroFLOW has shown promising results. It has also been shown to improve crop yields in agriculture, and in ice machines HydroFLOW facilitates the production of cleaner and clearer ice.
This technology has also shown that it will assist in controlling algae and bacteria in many different situations, for example it will reduce your pool chlorine usage and still provide pathogen control. HydroFLOW is proving itself in the power generation industry as a valuable asset and has a long-standing record of providing fantastic benefits to any industry that uses water and is having problems with scale, algae, corrosion or bacteria. In a world where chemicals have dominated as being the only solution, this physical water conditioner is a viable alternative. We as humans are getting smarter and have found better ways to solve problems than polluting our environment with chemicals and waste.
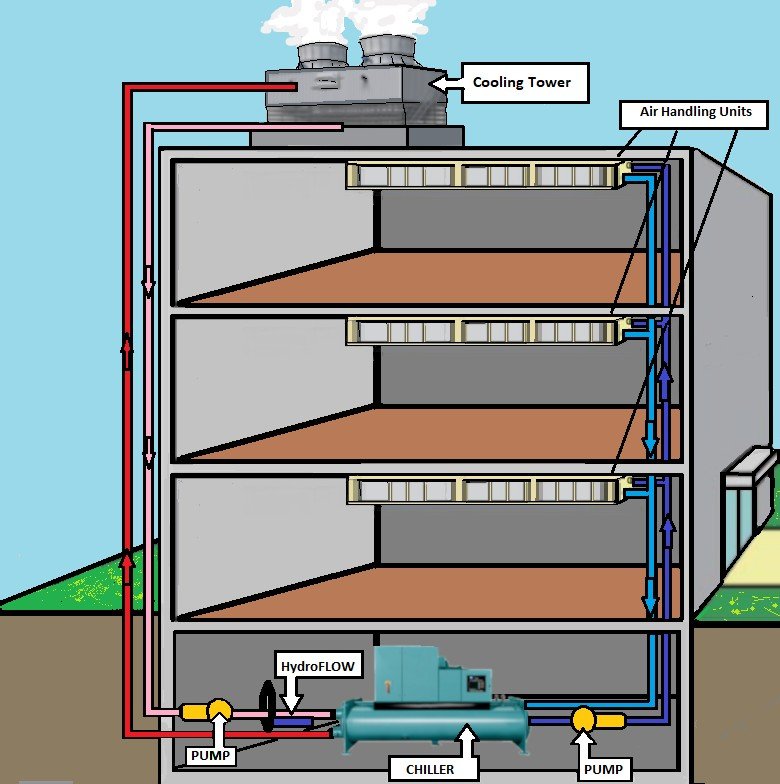
Cooling Towers
Cooling towers range in size and purpose from dealing with air conditioning in hotels to cooling power stations. However, they all perform the same basic task: they take heat from somewhere it is not wanted and dump it into the atmosphere. To do this they use water, for two main reasons 1) water is abundant and 2) water is very good at storing heat compared to other substances. If we are using water to cool something down, that means heat is being transferred from that something to the water, i.e. the water is being heated. Whenever water is heated, there are likely to be problems with limescale.
The basics of a cooling tower system that apply to almost every cooling tower in operation.
- Cooling towers keep a heat source cool by evaporating water
- Heat is transferred to the tower water in a heat exchanger
- Problems: limescale in heat exchangers, bacteria, and or algae in the tower water
- Blow-down/filtration and a chemical regiment are normally required for the good operation of the tower.
Cooling Tower Terminology
We now briefly note some of the terms commonly used in referring to cooling towers.
- Casing or shell – This is the structure that supports the rest of the components.
- Air Inlet and Outlet – The points at which air enters and leaves the tower.
- Fan In mechanical Draught Tower – a fan is provided to move the required amount of air through the water to be cooled.
- Water Inlet – This is the point at which water enters the tower.
- Water Distribution System – For maximum effect, the water entering the tower must be spread evenly over the top of the packing. This is what the distribution system does. There is a variety of distribution systems used, such as sprays or "weirs" where the water spreads by gravity.
- Drift Loss- is the water that leaves the cooling tower as droplets of water and typically equates to .3 gallons of water per ton of cooling capacity in a tower without drift eliminators. Additional water is used in a tower through evaporation. The rule of thumb being 3 gallons of water per ton of cooling capacity is used.
- Wet Bulb Temperature – Since cooling tower cells cool water by evaporation, the wet-bulb temperature is the critical design variable. That means that if the wet-bulb temperature is 78°F, then the cooling tower will most likely provide cooling water between 83° – 85°F, no lower.
The main point of a cooling tower is to cool something down. The process of cooling down requires more than just a cooling tower. The next important piece of a cooling tower system is the heat exchanger. Tube in shell exchangers is often used in larger towers. In this type of exchanger, the tower water passes through a series of pipes within a “shell" or metal casing the heat source (the steam or water that the tower is supposed to be cooling) passes through the shell around the tubes. Alternatively, the tower water can pass through the shell, and the heat source can pass through the tubes. In either case, the scale will form on the surfaces that are in contact with the tower water.
What is a Heat Exchanger?
Obviously, the name “heat exchanger” implies that there is a transfer of heat taking place. This assumption is in fact true. It is the piece of equipment that the transfer of heat takes place. The heat exchanger is where water absorbs heat energy from the heat source, so the heat source is cooled, and the tower water is heated. The water is then pumped to the top of the cooling tower, where it is sprayed in a fine mist down through the tower. As the water drops fall through the air, they are cooled (mostly by evaporation). The water collects in a pool at the bottom of the tower (a tank or sump), and from there it is then pumped back to the heat exchanger, to start the process again. In a cooling tower system, they might be using what's called a condenser instead of a heat exchanger. So, what is the difference between a condenser and a heat exchanger?
Difference Between a Condenser vs Heat Exchanger?
When the heat source that the tower is cooling is steam rather than hot water, the heat exchanger is referred to as a condenser, although they are basically the same. This is because the steam is condensing back from steam to water. The same basic principles apply with both water and steam heat sources. One extra difficulty with steam sources is that due to the high temperature of the steam, the tower water can boil at certain points (called “nucleate boiling" or “kettling"). This will leave all of the dissolved minerals behind, and so it is very hard to treat water for limescale if this type of boiling occurs inside the heat exchanger.
One of the main problems is the limescale in the heat exchangers. As we know, limescale tends to form when water is heated (as the water becomes less able to hold onto the ions) and so the heat exchangers (where the tower water is heated) are where we find the scale. This makes the cooling tower less efficient, as the limescale acts as thermal insulation, making it harder for the heat to get from the heat source to the tower water. Another significant problem is biofouling, or bacteria and algae. These will often grow in the tower pool, which is a warm, stagnant body of water and therefore ideal for the growth of microorganisms. Both limescale and biofouling are commonly dealt with by the addition of chemicals (anti-scalant and disinfectant). These are costly, need continual replacement, and can harm the environment.
What is Blowdown and Why is it Necessary on a Cooling Tower?
As the water is gradually evaporating to cool down, it gets more and more concentrated over time. The water is sprayed through the air to cool it and will pick up dust and other debris. The common way to deal with the build-up of minerals and debris in the cooling tower is to perform a blowdown. What does blowdown mean? “Blowdown" is a term meaning that the water in the tower pool is flushed away and replaced with new water. The minerals, dirt, and suspended solids are washed away with the discarded blowdown water. This water must be replaced – this replacement water is called makeup water.
Cooling towers work much more effectively with an automatic blowdown system installed, which discard and replace some of the water automatically whenever a certain concentration of dissolved solids is reached. Manually operated blow-down systems are not always operated optimally and can cause problems. Manufacturers will usually have recommendations about the blow-down level. Sticking to these limits will certainly be enough to ensure that there is no excessive build-up of calcium carbonate crystals. Blow-down rates can be reduced below the manufacturers' limits from a scaling point of view (as long as a reasonable amount is still performed) but care needs to be taken as scaling is not the only potential problem with highly concentrated water. These will often be in terms of “cycles of concentration”.
What Does “Cycles of Concentration” Mean?
Cycles of concentration are how many times more concentrated the tower water is than the make-up water. If no manufacturer's instructions are given, then a safe rule of thumb is that blow down is performed often enough to keep the concentration of the water (the T.D.S.) at no higher than 2000 ppm or no more than 3-4 times that of the incoming water (for very hard water). In some situations, the water from the cooling tower is subsequently used for another purpose – for instance, in a factory the water may later be used for some industrial process, or in a hotel it may be used as “grey water," i.e. for flushing toilets, etc.
Using HydroFLOW In these cases, as long as sufficient water is being used that the concentration of the water and the number of suspended particles do not get too high, additional blowdown will not be necessary – we are effectively already doing blow down but just using the blowdown water for something rather than throwing it away!
Is Filtration Needed with a Cooling Tower?
Filtration can also help remove the larger particles from the tower pool, which in turn reduces the amount of blowdown required. The type of filtration used in cooling towers is called side stream filtration which means that, rather than being fitted on the main pipe, the filter is fitted to a smaller side stream pipe that takes water out of the pool, filters it, and then returns it to the pool. The rate at which the water should be filtered depends on the rate it is passing through the tower – if the filtration rate is very slow, then all the water will be sent to the tower again before passing through the filter! A good rule of thumb is that the flow rate of the side stream should be around 10% of the main flow rate.
The filter has to be periodically back washed, i.e. water is forced in the opposite direction to flush out all the accumulated debris, which is then discarded. Again, we recommend that this backwashing is performed automatically.
What is a Steam Boiler?
A steam boiler or steam generator is an apparatus designed to convert water into steam by heating it. It is therefore essentially a kind of heat exchanger, where the water on one side is heated by some heat source, usually the exhaust gasses from the combustion of some fuel. The steam can be used for a range of applications, including in garment factories for ironing and paper mills, and the pressurized steam can also be used to drive machinery. Sometimes the steam is fully used up (such as in the ironing application) but other times it can be fully or partially recycled. After passing through the system and doing its job, the steam will cool down and turn back into the water. It can then be returned to the boiler for re-heating.

What is it Meant by a Closed-Loop System?
In a fully closed loop system, all the steam is condensed back to water and returned to the boiler after use. In theory, this means that no new water ever enters the system, and hence there should be minimal scale problems. Of course, no system is perfect and so it will need new water added to top up the system – makeup water. It might be thought that the best water to use would be completely demineralized or distilled water so that there are no scale problems.
However, distilled water is extremely corrosive and so in practice, the water is often softened rather than distilled, and there are anti-corrosion chemicals added. As the water is continually evaporating, the concentration of the chemicals will be liable to increase. This means that the balance and concentration must be carefully monitored and adjusted. These complications, and the fact that the softening of the water may not be done perfectly, means that scale problems can occur even in closed systems.
What is the Difference Between an Open Loop System vs Closed Loop System?
If the system is not closed loop, i.e. the steam is being used up or lost in significant quantities, and then new water will need to be added to the system to replace the losses. If there is a substantial amount added, it will usually be too expensive to use demineralized water. Instead, the make-up water will often be softened via an ion exchange softener (salt or resin). This leads to the obvious expense of having to continually supply chemicals to the ion-exchanger. An alternative method of treating the make-up water is reverse osmosis, but again this is expensive.
Is a Steam Boiler the Same Thing as a Heat Exchanger?
A steam boiler can be seen as a kind of heat exchanger. The main difference is that while it is water being heated in both cases (heat exchanger and steam boiler), in a steam boiler it will be extremely hot exhaust gasses doing the heating. As with heat exchangers, steam boilers fall into two broad categories: Fire Tube and Water Tube.
What is the Difference Between Water Tube Boilers and Fire Tube Boilers?
These boilers consist of a steel drum, through which passes a series of tubes. Hot exhaust gasses pass through the tubes and heat water that is in the steel drum around them. The steam then collects at the top of the drum. Fire tube boilers are relatively easy to operate and install and are therefore used in small installations to heat buildings and power factory processes. Steam locomotive engines are also essentially fire tube steam boilers, where the steam is used to drive pistons to turn the wheels.
Water-tube boilers are designed to provide larger amounts of high-pressure and high-temperature steam. The water flows inside the tube, and the steam thereby generated is stored in a small drum. The smaller diameter of the tubes and the steam drum means that the structure is stronger and can therefore withstand higher pressures than fire-tube boilers. Units of substantial size are used in steel mills, paper mills, oil refineries, chemical plants, and other large manufacturing plants.
What is the Exhaust Temperature?
When discussing heat exchangers, we introduced the concept of Delta T, i.e. the temperature change between the incoming and outgoing water. We noted that this could be for either the heat source or the “cooling water”, and that we would like to have as big a temperature change (i.e. as big a Delta T) as possible. Now for a steam boiler, the equivalent of the “cooling water” is the water that is being converted to steam. In this situation, measuring the Delta T is not a useful measurement, as the water is being converted to steam at 100 degrees C – greater efficiency could lead to an increased volume of steam rather than increased temperature. The alternative is to measure how much the heat source is cooling down as it transfers its heat to the water. On a steam boiler, this corresponds to measuring the exhaust temperature of the hot gasses. A lower exhaust temperature means that the hot gas has transferred more energy to the water, i.e. a lower exhaust temperature means a more efficient transfer of heat.
In steam boilers, water is rapidly heated to high temperatures and evaporated with increasing concentration. This means that the water becomes supersaturated, and the ions in the water will be expelled to form limescale. Treating the water with Hydropath technology will cause the scale to form as small particles in suspension rather than the hard scale on the heating surfaces. It is important to note that this is not a chemical change, so any chemical measurements of the water quality will detect no change due to Hydropath.
I Need Help Protecting the Boiler from Scale Issues
HydroFLOW devices use the Hydropath technology and is an eco-friendly solution that works by causing the ions to form clusters which then turn into small crystals that attach to each other instead of your pipes and equipment. However, these clusters are rather delicate and can be broken up by intense turbulence, such as are found in high-pressure pumps. This means that in order to treat scale, we have to fit the unit after the pump. This is an important point to remember when selecting an installation point.
Protecting the Deaerator/ Deoxygenation Tank from Scale
One common way to attempt to reduce the amount of corrosion within steam boilers is to remove as much oxygen from the water as possible. To remove the dissolved oxygen, the water is placed in a tank (called a deaerator or deoxygenation tank) and some of the steam produced is bubbled through it (this steam maybe post use as steam). This helps release the dissolved oxygen and has the effect of preheating the water before it goes into the steam boiler itself. Sometimes the water is preheated anyway even if the water is not being deoxygenated. In both cases, the water is being heated before it even goes into the steam boiler itself. This means that there may be additional limescale problems, for example in the deoxygenation tank, as well as in the actual steam boiler. In these cases, an additional HydroFLOW unit should be fitted immediately before the deoxygenation tank or wherever the water is being heated.
Do I Need to Blow Down my Boiler?
In steam boilers, the water is being boiled away, leaving any dissolved minerals behind. Although Hydropath technology ensures that these minerals do not form hard limescale deposits, the tiny crystals produced will still build up. If they are allowed to build up for long enough, they will begin to cause a problem. This means that regular blow-down of the boiler is essential. The water in the boiler needs to be flushed out. This will ensure that the crystals formed from the ions in the water are removed from the system and do not build up to a point where they become problematic. If the boiler is initially scaled, then as the action of the HydroFLOW dissolves the existing scale, more minerals will be released into the water. The blowdown rate should be increased initially during this period.
Blowdown water is often monitored to assess how well the system is working. Two common parameters measured are the TDS and the level of the iron in the water. The TDS measures the number of minerals in the water, and so is normally taken as a measure of how much scaling there will be. The level of iron in the water is similarly taken as a measure of the corrosion rate. It is important to note that when HydroFLOW is fitted to an initially scaled boiler, the existing limescale will dissolve and the TDS will increase at first.
Limescale Prevention
The induced field causes crystals to form in suspension rather than as a hard deposit on the heat exchanger. By placing a unit before the heat exchanger, we can keep it clear of scale. However, we can only dissolve the existing scale if it is due to calcium carbonate and not silicates. Silicates are a set of ionic compounds that contain silicon. As they are ionic, the Hydropath signal affects them in the same way, but once the crystals are formed, they are very hard to remove. When silicates form on a pipe, they leave a smooth hard deposit, almost as if the inside of the pipe has been coated with glass. It may be worth testing the water to ensure that it is calcium carbonate causing the problem.
Agriculture
Another industry that we have proven our product is a valuable component to is agriculture. Scale is an issue for farmers that in the past was handled with chemicals. Now that being organic has become very important other means to treat a host of problems plaguing the agricultural sector. It should be noted that HydroFLOW has been certified for use on organics.
How can HydroFLOW help? By fixing scale issues in irrigation systems to algae and bacteria in holding ponds. HydroFLOW has had the opportunity to be installed at several large agricultural producers and we have seen fantastic results. Not only have we seen holding ponds cleaned up of algae and scale no longer being a problem for their irrigation systems we have seen that HydroFLOW treated water penetrated soil further, increased crop yields and a host of other benefits. We are still analyzing and investigating what the mechanisms that are producing these fantastic results. Stay tuned, since we have a few universities performing studies we should have the answers to these questions in the near future. As we discover the mechanisms, we will update our information. Truly an exciting time and new frontier for HydroFLOW.
Ice Machines – Does HydroFLOW really make ice clearer?
A slightly unusual application is the treatment of ice-making machines. There are two main effects – scale prevention and improving ice clarity.
Because scale can form when water is cooled (actually when it is frozen) rather than when it is heated. In practice however, there is no difference how we treat the system.
When water forms ice, it can expel the scale forming ions and leave limescale deposits on ice making machines. We can treat the water using Hydropath technology in exactly the same way as usual, by applying an electric field to the water before it is frozen. The field causes clusters to form, which will then cause the scale to form in suspension rather than on surfaces.
Ice clarity will also be improved. When ice is produced, it looks much nicer if the ice itself is clearer and more transparent. One cause of cloudy ice is bubbles, but another is that the ice forms as many different crystals.
As the ice cools down it begins to freeze, i.e. it changes from a liquid state to a crystalline state. Usually, the ice will begin to form crystals at lots of different places, which will then grow and join up. This means that the ice produced is formed up of many different small crystals, all oriented at random angles to each other. These joins will tend to scatter the light, making it look less clear.
Now, when any crystal form it needs a nucleation center – a starting point. In water forming ice, this could be some dirt, or bubbles, or anything else. However, when we apply a HydroFLOW unit, we are providing the water with a really good nucleation center – the crystal of limescale. these crystals acts as seeds for the ice formation, and make it easier for the ice crystals to form. Because it is easier for the ice to form, we actually find that the water freezes slightly sooner – at a slightly higher temperature – than it otherwise would. The point at which the ice forms can change by around 0.1 deg C. This is not really significant in terms of making the ice but clarifies what is happening. Now, because we have a few very good seed points, we end up with ice that is formed of fewer, larger crystals than we otherwise would have. This means that the ice formed is clearer and more attractive.